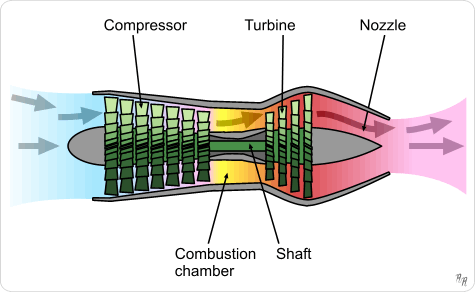
As title say.
Let's take a stock military turbojet such as the J-75.
Can it withstand H2O2 in its kerosene ?
Why such a strange question ? ;D
It's just that I'm currently interested in the Black Horse/ Black Colt / P.R Pathfinder concepts.
They used turbofans and H2O2 in-flight refueling (not all of them, there have been lots of variants from 15 years now)
Btw correct me if I'm wrong
- Seems that kerosene and H2O2 can be mixed into a single tank giving what is called a monopropellant... ???
- when LOX and hydrogen need separated tanks
(I suppose its because these cryogenics fuels have different boiling temperatures ?)
Let's take a stock military turbojet such as the J-75.
Can it withstand H2O2 in its kerosene ?
Why such a strange question ? ;D
It's just that I'm currently interested in the Black Horse/ Black Colt / P.R Pathfinder concepts.
They used turbofans and H2O2 in-flight refueling (not all of them, there have been lots of variants from 15 years now)
Btw correct me if I'm wrong
- Seems that kerosene and H2O2 can be mixed into a single tank giving what is called a monopropellant... ???
- when LOX and hydrogen need separated tanks
(I suppose its because these cryogenics fuels have different boiling temperatures ?)