You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Zero-carbon fuel PN (Calum?)
- Thread starter Pasoleati
- Start date
Without knowing the specifics of the fuel, there's no way to say. In principle you could synthesize any hydrocarbon. Petrol and diesel are both blends of a bunch of different hydrocarbons, plus other additives, and the octane number is determined by the exact composition.
Porsche hasn't published an octane rating goal, but it should be close to production petrol to make switching between the two easy.
Porsche hasn't published an octane rating goal, but it should be close to production petrol to make switching between the two easy.
- Joined
- 27 May 2008
- Messages
- 1,179
- Reaction score
- 2,485
Producing hydrocarbons from air I.e CO2 at 400ppm, and water at petroleum scale, while possible requires a mind boggling enormous amounts of energy and infrastructure construction, <To remove a giga ton of CO2/year needs an air intake 10m high by 5000km long, - enough for 300million tons of fuel—the EU uses approx 1500 million ton per year > . With the current technology it’s horribly inefficient such that would need the entire present day European electrical generating capacity. But to answer your question;- In theory if undertaken, any grade/octane rating can be produced with absolutely no impurities.
- Joined
- 19 July 2016
- Messages
- 4,287
- Reaction score
- 3,466
In a time where mining for %*it coin uses the electric generation sufficient to run Argentina or similar, it's not that far out of reach.
It actually not as ineffiecient as it seems, Methan and Methanol can be produced by direct air capturing with an efficiency of about 50 % and for Ammonia the efficiency is much higher. Using surplus power on weekends or during the nighttime could be an intresting option.
Thermal depolymerization seems a better approach. Take sewage, lawn trimmings, biowaste of all kinds, run through the machine, petroleum comes out the other side. Requires feedstock that can be either garbage or grown specifically (and unlike corn-fed ethanol, the crop need not be a *food* crop, weeds will work just fine), and energy. Energy can come in the form of electrical input, or by burning some of its own product.
Apparently one of the biggest problems the system encountered, and what led to failures of some of the startups, is that the process *stinks.* Not such an issue if you plan ahead.
Apparently one of the biggest problems the system encountered, and what led to failures of some of the startups, is that the process *stinks.* Not such an issue if you plan ahead.
- Joined
- 27 May 2008
- Messages
- 1,179
- Reaction score
- 2,485
It actually not as ineffiecient as it seems, Methan and Methanol can be produced by direct air capturing with an efficiency of about 50 %
My information comes from this assessment of “Carbon Engineering Ltd” (Canada) Direct Air Capture Process by Howard Herzog an Process Engineering lecturer at MIT.
Note 1 Howard is referring to the extraction of CO2 from air and not passing comment on which fuel source it’s being converted into.
From the information above and others the overall energy conversion efficiency ie energy in to energy stored, for a DCC derived fuel is probably lower than 5%.
Ammonia is derived from atmospheric nitrogen which present in about 80% of air so doesn’t make a fair comparison.
Please advise where he’s going wrong and provide a source for your 50% figure.
Shell to build one of Europe’s biggest biofuels facilities for SAF and renewable diesel; 820,000 tonnes/year
Royal Dutch Shell will build an 820,000-tonnes-a-year biofuels facility at the Shell Energy and Chemicals Park Rotterdam, the Netherlands, formerly known as the Pernis refinery. Once built, the facility will be among the largest in Europe to produce sustainable aviation fuel (SAF) and renewable...
www.greencarcongress.com
Kinder Morgan and Neste partner on major renewable fuels logistics project in US
Kinder Morgan, one of North America’s largest energy infrastructure companies, is partnering with Neste, one of the leading providers of renewable and circular solutions, to create a premier domestic raw material storage and logistics hub in the United States, supporting increased production of...
www.greencarcongress.com
Marathon Petroleum confirms successful test run for US refinery producing 100% renewable diesel based on Topsoe’s HydroFlex
In late 2020, Marathon Petroleum Corporation (Marathon) began the production of renewable diesel at its Dickinson refinery in North Dakota. Marathon now reports successful completion of the refinery’s test run of the grassroot renewable diesel unit, based on Topsoe’s HydroFlex technology...
www.greencarcongress.com
I have no special knowledge worth sharing on this question, as I have not looked into it. 
So I should (currently) be considered clueless on this specific point on the Porsche fuel.
However, for the sake of trying to say something vaguely useful on a more generic level:
Looking in general at the history of synthetically produced fuels, there is usually a "PN/production volume"
mutual exclusivity function at work. I`m deeply sceptical of most "green" stuff as in my personal view,
I`ve yet to find one which once you add up the hidden costs and lifecycle issues, doesnt end up
"robbing Peter to pay Paul". However, my "bah-humbug" opinions aside, I would also think that this
stuff ougt to be pretty decent in anti-knock rating to make the engines which use it of small a size
as possible and to enable maximum CR (which is the basic function of efficiency at the root
of all engines).
That said, modern understanding of combustion systems (the layout of the combustion chamber, plug,
Injector, ports etc) already means that with 99 RON you can get from many perfectly ordinary petrol stations
here (shell V-power) its possible to run 4-bar boost pressure without any knock. If you`re using a really
well designed modern engine, the rationale for investigating ultra high knock-resistant fuels, just isnt there,
you can already get to the point of blowing the bearings out the bottom with fuels with un-spectacular
anti-knock rating.
So I should (currently) be considered clueless on this specific point on the Porsche fuel.
However, for the sake of trying to say something vaguely useful on a more generic level:
Looking in general at the history of synthetically produced fuels, there is usually a "PN/production volume"
mutual exclusivity function at work. I`m deeply sceptical of most "green" stuff as in my personal view,
I`ve yet to find one which once you add up the hidden costs and lifecycle issues, doesnt end up
"robbing Peter to pay Paul". However, my "bah-humbug" opinions aside, I would also think that this
stuff ougt to be pretty decent in anti-knock rating to make the engines which use it of small a size
as possible and to enable maximum CR (which is the basic function of efficiency at the root
of all engines).
That said, modern understanding of combustion systems (the layout of the combustion chamber, plug,
Injector, ports etc) already means that with 99 RON you can get from many perfectly ordinary petrol stations
here (shell V-power) its possible to run 4-bar boost pressure without any knock. If you`re using a really
well designed modern engine, the rationale for investigating ultra high knock-resistant fuels, just isnt there,
you can already get to the point of blowing the bearings out the bottom with fuels with un-spectacular
anti-knock rating.
There is something called the synhelion I read about recently…to produce carbon fuels…and a recent phys.org blurb about capturing CO2 and water from automobiles for crops. In terms of aviation…Reaction engines was looking at ammonia partially cracked into hydrogen. Instead of an ammonia NTR…perhaps an atomic pile can split the ammonia with nickel and electricity with the hydrogen going to regular combustion and the nitrogen released to jacket the thrust to reduce thermal signatures…elaborate 3D printed tubing will open a lot of things up I hope…allowing for ammonia wet wings and eliminating Skylon sized tankage…an NTR that isn’t an NTR?
Sorry for not neither having an english quotation nor the original origin (maybe I will find it again)
For the efficiency of the chemical process, you need to divide the last collum with the second last one (of course 55% means 0.55).
So for Ammonia you get an efficiency of 66 % and for DME only 46 %. The complets process with ammonia as fuel beeing used in a combustion engine is as effective as liquified hydrogene beeing used in a fuel cell. Ammonia is much easier to transport and store and the investment cost are way lower than in an hydrogene infrastructure.
For the efficiency of the chemical process, you need to divide the last collum with the second last one (of course 55% means 0.55).
So for Ammonia you get an efficiency of 66 % and for DME only 46 %. The complets process with ammonia as fuel beeing used in a combustion engine is as effective as liquified hydrogene beeing used in a fuel cell. Ammonia is much easier to transport and store and the investment cost are way lower than in an hydrogene infrastructure.
Attachments
Last edited:
- Joined
- 27 May 2008
- Messages
- 1,179
- Reaction score
- 2,485
I don’t understand the figures in your table and where the table itself comes from.
The process used to convert CO2 and hydrogen into a fuel is the Fischer Tropsch.This process runs at 1100C so is vey energy intensive.
In simple terms, if the maximum theoretical thermodynamic efficiency of Fischer Tropsch is 51%< see link below & remember the real world will be less than this> then the final efficiency will further diminish with each step in the fuel production;- Carbon Capture Air induction,CO2 separation, transport, hydrogen generation, electrical power generation etc hence the final energy fraction will be very significantly less.

 www.sciencedirect.com
www.sciencedirect.com
The process used to convert CO2 and hydrogen into a fuel is the Fischer Tropsch.This process runs at 1100C so is vey energy intensive.
In simple terms, if the maximum theoretical thermodynamic efficiency of Fischer Tropsch is 51%< see link below & remember the real world will be less than this> then the final efficiency will further diminish with each step in the fuel production;- Carbon Capture Air induction,CO2 separation, transport, hydrogen generation, electrical power generation etc hence the final energy fraction will be very significantly less.

Process efficiency of biofuel production via gasification and Fischer–Tropsch synthesis
A thermodynamic equilibrium model was used to predict the composition of syngas produced by oxygen-blown biomass gasification at different operating c…
I have to agree, that my tables are not self explaining, tha last collum is giving the total efficiency from electricity to fuel to electricity by a fuel cell. The colum before is the efficiency of the fuel cell working with that specific fuel. To calculate the efficiency from electricity to fuel you have to divede the total efficiency by the fuel cell efficiency. The tables are taken from a puplication of the DLR (I'm quite shure about it) but I don't remember the original source.
Youre example of the Fisher Tropsch process ist the conversion of biomass to liquid fuel, this is not simillar to convert CO2 + H2O to Methanol or Methan or any other syn. fuel.
Youre example of the Fisher Tropsch process ist the conversion of biomass to liquid fuel, this is not simillar to convert CO2 + H2O to Methanol or Methan or any other syn. fuel.
- Joined
- 25 June 2014
- Messages
- 1,564
- Reaction score
- 1,499
Is zero-carbon petrol really going to be a thing?
The global motor industry are racing like lemmings for the cliff of all-electric. Where electric falls short is in the long-distance grind, and here fuel oil rules unchallenged. The road user knows the lighter grades of fuel oil as Diesel, but heavier grades - bunker oil - are burned by the world's shipping. Indeed, so-called marine diesels (including many derived from the Junkers two-piston two-stroke Diesel aero engines, some even via the Napier Deltic) do not burn Diesel fuel but bunker oils. How long can a zero-emission petrol hold its niche ahead of the electric race?
Then again, I would argue that petrol is a false goal. Diesel for road use is currently out of favour, due to its higher emissions of carcinogenic particulates and nitrogen oxides. These hurt the current generations but are gone in a few decades. On the other hand petrol has significantly higher CO2 emissions, and this stuff hangs around for thousands of years, cooking the world of our childrens' children's children unto the [a lot more than seventh] generation. Clearly, if we are serious about combating climate change, Diesel is the friendlier choice.
Sorry Porsche, you don't get my vote.
The global motor industry are racing like lemmings for the cliff of all-electric. Where electric falls short is in the long-distance grind, and here fuel oil rules unchallenged. The road user knows the lighter grades of fuel oil as Diesel, but heavier grades - bunker oil - are burned by the world's shipping. Indeed, so-called marine diesels (including many derived from the Junkers two-piston two-stroke Diesel aero engines, some even via the Napier Deltic) do not burn Diesel fuel but bunker oils. How long can a zero-emission petrol hold its niche ahead of the electric race?
Then again, I would argue that petrol is a false goal. Diesel for road use is currently out of favour, due to its higher emissions of carcinogenic particulates and nitrogen oxides. These hurt the current generations but are gone in a few decades. On the other hand petrol has significantly higher CO2 emissions, and this stuff hangs around for thousands of years, cooking the world of our childrens' children's children unto the [a lot more than seventh] generation. Clearly, if we are serious about combating climate change, Diesel is the friendlier choice.
Sorry Porsche, you don't get my vote.
Electric already has the range. Those who hold oil stocks are desperate to see prices go up. In the meantime, the oil industry will continue since a great deal of money has to be shifted from oil to alternatives. It appears all of that has been planned out.
- Joined
- 19 July 2016
- Messages
- 4,287
- Reaction score
- 3,466
There are already liquid fuel alternatives to those currently in use. Why so many are fixating on BE as a aole method is beyond me. We are missing a trick to cut emissions now.
The synthetic fuels can be divided into three efficiency catagories:
1. Ammonia and Hydrogen (most efficient since no carbon needed)
2. Methan and Methanol (second most efficient since synthesis is relative easy)
3. all other Hydrocarbons (Jet fuel, Gazoline, Diesel....)
Im pretty shure, there will be Jet fuel (more or less the same as Diesel fuel) availeble, there is almost no alternative for very long range and military aplications. Most GA planes are operated with 100 LL, I doubt that someone will make synthetic fuel containing lead.
The efficiency of synthetic fuels might be low, but dont forget, you can use surplus electric energy, e.g. from wind generators on weekends or nuclear power stations during the night. Despite we have about 40 % regenerative electricity in Germany, we often have surplus energy left and turn the wind generators out of the stream, because there is no use for this electricity. Some countries like france operate their nuclear power station according to the electricity demand, but reducing the power output of an nuclear power station hardly helps to save any money at all.
1. Ammonia and Hydrogen (most efficient since no carbon needed)
2. Methan and Methanol (second most efficient since synthesis is relative easy)
3. all other Hydrocarbons (Jet fuel, Gazoline, Diesel....)
Im pretty shure, there will be Jet fuel (more or less the same as Diesel fuel) availeble, there is almost no alternative for very long range and military aplications. Most GA planes are operated with 100 LL, I doubt that someone will make synthetic fuel containing lead.
The efficiency of synthetic fuels might be low, but dont forget, you can use surplus electric energy, e.g. from wind generators on weekends or nuclear power stations during the night. Despite we have about 40 % regenerative electricity in Germany, we often have surplus energy left and turn the wind generators out of the stream, because there is no use for this electricity. Some countries like france operate their nuclear power station according to the electricity demand, but reducing the power output of an nuclear power station hardly helps to save any money at all.
Dagger
ACCESS: Secret
- Joined
- 24 December 2019
- Messages
- 335
- Reaction score
- 623
The only sensible zero-carbon "gasoline" would be pure bio-ethanol. Is already used in Brazil.Since there are projects attempting to produce zero-carbon petrol (diesel too?) by Porsche at least, I wonder what octane/PN rating would such petrol have?
Octane number is above 120.
At the moment ethanol can only be produced by fermentation of sugars. There is research already going on to also ferment cellulose into ethanol. That would be an enormous boost for bio-ethanol production.
Capturing CO2 from the atmosphere and react it with H2 (hydrogen) to produce hydrocarbon fuel is total BS, no matter what some "professor" from some "university" claims. It's merely subsidized hobbyism.
The cost of synthetic fuel is mainly the cost of energy. For 1 kg Diesel/Jet/Gazoline fuel (about one quart) you need in theorie about 12 kwh. When using nuclear energy or wind energy in southern America, the cost for 1 kwh is about 0,4 cent (Euro or Dollar is estimated to be roughly the same...). With an efficiency of 40 % the cost of energy will be around 1.2 €/kg or 1 €/L. That might be considered as high in the US, but certainly not in the EU. If only surplus energy is used, the cost will be much lower, so it is not entierly unrealistic.
Ammonia is also my favourite (as well as for many naval companies) but for aircrafts its lower energy densety compared to Kerosin is a drawback. At least for military aircrafts synthetic Kerosin will be used and I guess also for many GA applications.
Cracking of Ammonia will need a considerable amount of weight, but it would be suffiecient to crack only a small fraction of it. Ammonia powerd busses and cars were used decades ago, combustion engines can use this fuel und turbines propably also (the complete combustion might be difficult in a turbine).
Phys.org had a bit where the heat needed to crack ammonia was lessened:
“Breaking ammonia: A new catalyst to generate hydrogen from ammonia at low temperatures” from ACS Catalysis and Masaaki Kitano. Combine this with US patent 8778293 of Roger Gordon’s Green NH3…and you really have something.
Air and water to ammonia…then that to hydrogen. Less energy than getting hydrogen directly out of the water itself perhaps. See “Novel technique seamlessly converts ammonia to green hydrogen” also from phys.org
Guntae Kim has it that only 0.06 V is needed vs water needing 1.23 V
Another phys.org article is “captured water, carbon dioxide from car exhaust could help grow food.” Texas A&M. —from North Carolina State University: “Study finds plants would grow well in solar cell greenhouses.”
I see all this tech and LOX plants at power plants near water. You combine everything into one super factory making food, ammonia, LOX, etc.
“Breaking ammonia: A new catalyst to generate hydrogen from ammonia at low temperatures” from ACS Catalysis and Masaaki Kitano. Combine this with US patent 8778293 of Roger Gordon’s Green NH3…and you really have something.
Air and water to ammonia…then that to hydrogen. Less energy than getting hydrogen directly out of the water itself perhaps. See “Novel technique seamlessly converts ammonia to green hydrogen” also from phys.org
Guntae Kim has it that only 0.06 V is needed vs water needing 1.23 V
Another phys.org article is “captured water, carbon dioxide from car exhaust could help grow food.” Texas A&M. —from North Carolina State University: “Study finds plants would grow well in solar cell greenhouses.”
I see all this tech and LOX plants at power plants near water. You combine everything into one super factory making food, ammonia, LOX, etc.
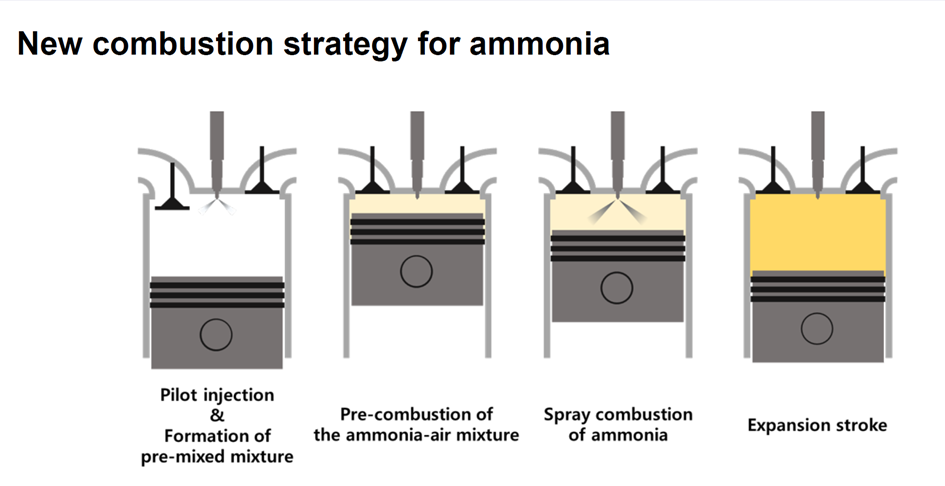
A new strategy for internal combustion of ammonia - Ammonia Energy Association
Of all the devices that can convert the chemical energy in ammonia to electricity, gas turbines and fuel cells appear to be receiving the lion’s share of development effort, outstripping that devoted to ammonia-fueled internal combustion engines (A-ICEs). An Ammonia Energy review last year...
 www.ammoniaenergy.org
www.ammoniaenergy.org
- Joined
- 15 January 2021
- Messages
- 402
- Reaction score
- 1,482
If you are going to make ethanol from either fermented sugars or cellulose, a better fuel is butanol. This 4 carbon alcohol has higher energy density, high Performance Number (100+), runs at similar fuel air ratios as gasoline, and doesn’t mix with water like ethanol.The only sensible zero-carbon "gasoline" would be pure bio-ethanol. Is already used in Brazil.Since there are projects attempting to produce zero-carbon petrol (diesel too?) by Porsche at least, I wonder what octane/PN rating would such petrol have?
Octane number is above 120.
At the moment ethanol can only be produced by fermentation of sugars. There is research already going on to also ferment cellulose into ethanol. That would be an enormous boost for bio-ethanol production.
Capturing CO2 from the atmosphere and react it with H2 (hydrogen) to produce hydrocarbon fuel is total BS, no matter what some "professor" from some "university" claims. It's merely subsidized hobbyism.
There are challenges to be overcome to ferment butanol from sugars but a lot of research has been done to get similar conversion efficiencies as ethanol fermentations. And cellulose fermentation has the same challenges for both.
lostcosmonauts
ACCESS: Confidential
- Joined
- 8 March 2020
- Messages
- 59
- Reaction score
- 79
The ABE fermentation has a long history (making cordite a century or more ago with the acetone and the butanol was essentially a byproduct)If you are going to make ethanol from either fermented sugars or cellulose, a better fuel is butanol. This 4 carbon alcohol has higher energy density, high Performance Number (100+), runs at similar fuel air ratios as gasoline, and doesn’t mix with water like ethanol.The only sensible zero-carbon "gasoline" would be pure bio-ethanol. Is already used in Brazil.Since there are projects attempting to produce zero-carbon petrol (diesel too?) by Porsche at least, I wonder what octane/PN rating would such petrol have?
Octane number is above 120.
At the moment ethanol can only be produced by fermentation of sugars. There is research already going on to also ferment cellulose into ethanol. That would be an enormous boost for bio-ethanol production.
Capturing CO2 from the atmosphere and react it with H2 (hydrogen) to produce hydrocarbon fuel is total BS, no matter what some "professor" from some "university" claims. It's merely subsidized hobbyism.
There are challenges to be overcome to ferment butanol from sugars but a lot of research has been done to get similar conversion efficiencies as ethanol fermentations. And cellulose fermentation has the same challenges for both.
Having spent a lot of effort on sourcing bio butanol for work I can say it is really really tough to get the economics to work and several start ups have failed in trying
Ammonia can easily be cracked by exhaust the heat of an internal combustion engine, as long as it is operated with medium to higher loads. It is not necessary to crack a large amount of ammonia, just 5 to 10 % will be sufficient to increase the flamability. No high tech materials will be needed and no electric energy.
Electric does not have the range. A Tesla can go about 400 miles on a charge with the highest tier battery. Then it needs to recharge for several hours. My full size, 4x4, V-8 powered pickup can go 430 miles on a tank and refuels in 5 mins from anywhere, including a jerry can. My range doesn't drop when I need heat since heat is a waste product in an IC engine. There are multiple real world reasons why battery powered vehicles don't work en mass. The previously mentioned usability, the complete lack of electric generation capability to power them, the lack of power transmission to get the power to them and the huge fire danger. A gas or diesel vehicle burns hot for a few mins. A lithium vehicle burns hot for hours and a warehouse full of the stuff burns for DAYS to WEEKS.Electric already has the range. Those who hold oil stocks are desperate to see prices go up. In the meantime, the oil industry will continue since a great deal of money has to be shifted from oil to alternatives. It appears all of that has been planned out.
Yeah, they are. I'm not trying to imply they are not. But lithium burns far, far longer. There was a warehouse fire in Chicago last summer. Took a week to bring it under control. I think they were still cooling it down after 2 weeks.Exploding gas tanks are really messy. Exploding gas tankers...
- Joined
- 6 November 2010
- Messages
- 5,264
- Reaction score
- 5,521
For the daily commute and shopping, BEVs have enough range. Haulage and holiday traffic are a different story.
A personal story: once, on a very long motorbike journey, I was nearly ready filling up when I noticed sparks falling around my feet. I had kept my crash helmet on, which had somewhat limited my awareness of my surroundings. Those sparks were caused by somebody doing some welding (or using a blowtorch?) up in the canopy of the filling station. I was too much in shock to do anything other than wrap up filling, pay the bill (for which I took off my helmet) and then carefully driving away. In the past, I hadn't witnessed any fuel explosions - just the results of them. A few Ks down the road I stopped to sort out the shakes that had suddenly started.
A personal story: once, on a very long motorbike journey, I was nearly ready filling up when I noticed sparks falling around my feet. I had kept my crash helmet on, which had somewhat limited my awareness of my surroundings. Those sparks were caused by somebody doing some welding (or using a blowtorch?) up in the canopy of the filling station. I was too much in shock to do anything other than wrap up filling, pay the bill (for which I took off my helmet) and then carefully driving away. In the past, I hadn't witnessed any fuel explosions - just the results of them. A few Ks down the road I stopped to sort out the shakes that had suddenly started.
They do. But the infrastructure to recharge millions of them at the same time just isn't there.For the daily commute and shopping, BEVs have enough range.
- Joined
- 19 July 2016
- Messages
- 4,287
- Reaction score
- 3,466
Not so much, charging centres need space which is at a premium in cities but, the intent is to get those users onto the already overcrowded public transport system. Something already unfit for purpose.
Not really. Let's assume you live in a small community of a hundred or so homes. You all go to work and come home in a roughly 2 hour time period. Plug your cars in to charge, now your hundred homes are pulling down megawatts of power. Now multiply that by thousands of other communities. The grid is built on an assumption of a relatively stable load.It depends on where you live.
Don't get me started on the absurdity of those two power sources. Staying with our electric car example, you CAN'T charge a car overnight with solar...The grid needs to be upgraded anyway for decentralised power generation. Wind turbines. Solar power.